This paper from March 2015 is a primer that anybody can understand, including you. The IoBNT is the final building block of the surveillance network, bridging all living things from the biochemical domain into the electrical domain of the Internet.
There was no warning that nanotechnology of this sort was being pumped into your veins when you received a mRNA injectable from Pfizer or Moderna. Not a word from the government, Big Pharma, or the Military. There was no Informed Consent offered. The non-stop propaganda blared “Safe and Effective.”

 Technocracy is literally on track to conquer the human race, while humans don’t have a clue that a war is being waged against them in the first place. This is the topic of OMNIWAR: A SYMPOSIUM on September 21. I will be participating with Catherine Austin Fitts, David Hughes, Daniel Broudy and Lissa Johnson. It is free but you should RSVP to get further notices. This is the first global livestream that will reach all corners of the world. Can you see why I am asking to shout it from the roof top? ⁃ Patrick Wood, Editor.
Technocracy is literally on track to conquer the human race, while humans don’t have a clue that a war is being waged against them in the first place. This is the topic of OMNIWAR: A SYMPOSIUM on September 21. I will be participating with Catherine Austin Fitts, David Hughes, Daniel Broudy and Lissa Johnson. It is free but you should RSVP to get further notices. This is the first global livestream that will reach all corners of the world. Can you see why I am asking to shout it from the roof top? ⁃ Patrick Wood, Editor.
The IoBNT stands as a paradigm-shifting concept for communication and network engineering, where novel challenges are faced to develop efficient and safe techniques for the exchange of information, interaction, and networking within the biochemical domain while enabling an interface to the electrical domain of the Internet.
Abstract
The Internet of Things (IoT) has become an important research topic in the last decade, where things refer to interconnected machines and objects with embedded computing capabilities employed to extend the Internet to many application domains. While research and development continue for general IoT devices, there are many application domains where very tiny, concealable, and non-intrusive Things are needed.
The properties of recently studied nanomaterials, such as graphene, have inspired the concept of Internet of NanoThings (IoNT), based on the interconnection of nanoscale devices. Despite being an enabler for many applications, the artificial nature of IoNT devices can be detrimental where the deployment of NanoThings could result in unwanted effects on health or pollution. The novel paradigm of the Internet of Bio-Nano Things (IoBNT) is introduced in this paper by stemming from synthetic biology and nanotechnology tools that allow the engineering of biological embedded computing devices.
Based on biological cells, and their functionalities in the biochemical domain, Bio-NanoThings promise to enable applications such as intra-body sensing and actuation networks, and environmental control of toxic agents and pollution. The IoBNT stands as a paradigm-shifting concept for communication and network engineering, where novel challenges are faced to develop efficient and safe techniques for the exchange of information, interaction, and networking within the biochemical domain, while enabling an interface to the electrical domain of the Internet.
Introduction
The Internet of Things (IoT) defines a cyber physical paradigm, where all types of real-world physical elements (sensors, actuators, personal electronic devices, or home appliances, among others) are connected, and are able to autonomously interact with each other. This new form of seamless connectivity is the enabler for many applications such as machine to machine communication, real time monitoring of industrial processes, smart cities, smart grids for energy management, intelligent transportation, environmental monitoring, infrastructure management, medical and healthcare systems, building and home automation, and large scale deployments. The Internet of Things became a focus for research and development in the last 15 years. A large amount of investments for Internet of Things was and is still being made by government agencies and industry worldwide.
Recently, the concept of IoT has been revised in light of novel research advances made in the field of nanotechnology and communication engineering, which enable the development of networks of embedded computing devices, based on nanomaterials such as graphene or metamaterials, having scales ranging from one to a few hundred nanometers, called nanothings. The Internet of NanoThings (IoNT), introduced for the first time in [1], is proposed as the basis of numerous future applications, such as in the military, healthcare, and security fields, where the nanothings, thanks to their limited size, can be easily concealed, implanted, and scattered in the environment, where they can cooperatively perform sensing, actuation, processing, and networking.
While nanothings can push the engineering of devices and systems to unprecedented environments and scales, similarly to other devices, they have an artificial nature, since they are based on synthesized materials, electronic circuits, and interact through electromagnetic (EM) communications [1]. These characteristics can be detrimental for some application environments, such as inside the body or in natural ecosystems, where the deployment of nanothings and their EM radiation could result in unwanted effects on health or pollution.
A novel research direction in the engineering of nanoscale devices and systems is being pursued in the field of biology, by combining nanotechnology with tools from synthetic biology to control, reuse, modify, and reengineer biological cells [2]. By stemming from an analogy between a biological cell and a typical IoT embedded computing device, a cell can be effectively utilized as a substrate to realize a so-called Bio-NanoThing, through the control, reuse, and reengineering of biological cells’ functionalities, such as sensing, actuation, processing, and communication.
Since cells are based on biological molecules and biochemical reactions, rather than electronics, the concept of Internet of Bio-NanoThing (IoBNT), introduced in this article, is expected to be paradigm shifting for many related disciplines, such as communication and network engineering, which is the focus of this article. The execution of DNA-based instructions, the biochemical processing of data, the transformation of chemical energy, and the exchange of information through the transmission and reception of molecules, termed molecular communication (MC) [3], are at the basis of a plethora of applications that will be enabled by the IoBNT, such as:
- Intra-body sensing and actuation, where Bio-NanoThings inside the human body would collaboratively collect health-related information, transmit it to an external healthcare provider through the Internet, and execute commands from the same provider such as synthesis and release of drugs.
- Intra-body connectivity control, where Bio- NanoThings would repair or prevent fail-ures in the communications between our internal organs, such as those based on the endocrine and the nervous systems, which are at the basis of many diseases.
- Environmental control and cleaning, where Bio-NanoThings deployed in the environment, such as a natural ecosystem, would check for toxic and pollutant agents, and collaboratively transform these agents through bioremediation, e.g. bacteria employed to clean oil spills.
This article is organized as follows. First, Bio- Nano-Things are defined in light of the tools available today from synthetic biology and nanotechnology. Second, the application of communication engineering to design Bio-NanoThings telecommuni- cations is detailed, while the challenges to engineer Bio-NanoThings networks and Internet connections are discussed. Third, we describe further research challenges for the realization of IoBNT. Finally, we conclude the article.
Bio-NanoThings
Within the scope of the IoBNT, Bio-NanoThings are defined as uniquely identifiable basic structural and functional units that operate and interact within the biological environment. Stemming from biological cells, and enabled by synthetic biology and nanotechnology, Bio-NanoThings are expected to perform tasks and functionalities typical of the embedded computing devices in the IoT, such as sensing, processing, actuation, and interaction with each other.
BIOLOGICAL CELLS AS THE SUBSTRATES OF BIO-NANOTHINGS 

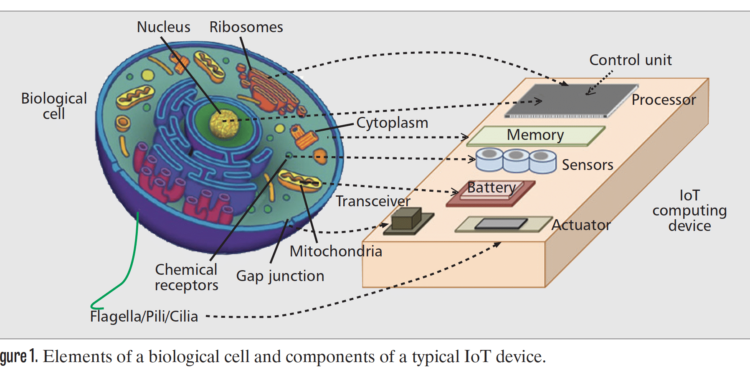
A biological cell is the basic unit of life, consisting of a membrane that encloses a mixture of highly specialized molecules, with defined chemical composition and function, which may also be organized into functional structures [4]. A mapping between the components of a typical IoT embedded computing device, and the elements of a cell, becomes apparent if we compare electrons’ propagation in semiconductors to functionally similar, although much more complex, biochemical reactions. In this context, as illustrated in Fig. 1, some examples are as follows.
The control unit, which contains the embedded software of the device, would correspond to the genetic instructions densely packed into the cells’ DNA molecules, which encode protein structures, the cell’s “data units,” and regulatory sequences, similar to the software conditional expressions.
The memory unit, which contains the values of the embedded system data, would correspond to the chemical content of the cytoplasm, i.e. the interior of the cell, comprised of molecules synthesized by the cell as a result of DNA instructions, and other molecules or structures, e.g. vesicles, exchanged with the external environment.
The processing unit, which executes the software instructions and manages memory and peripherals, would correspond to the molecular machinery that, from the DNA molecules, through the so-called transcription and translation, generates protein molecules with instruction-dependent types and concentrations.
The power unit, which supplies the energy to maintain the electrical currents in the embedded system’s circuits, would correspond to the reservoir in the cell of the Adenosine TriPhosphate (ATP) molecule, which is synthesized by the cell from energy supplied from the external environment in various forms, and provides the energy necessary for the cell’s biochemical reactions to take place.
The transceivers, which allow the embedded systems to exchange information, would correspond to the specific chains of chemical reactions, i.e. signaling pathways, through which cells exchange information-bearing molecules.
Sensing and actuation, which allow embedded systems to acquire data and interact with the environment, would correspond to the capability of a cell to chemically recognize external molecules or physical stimuli, e.g. light or mechanical stress, and to change the chemical characteristics of the environment or mechanically interact through moving elements, such as flagella, pili, or cilia.
ENABLING TECHNOLOGIES AND CHALLENGES
The discipline of synthetic biology is providing tools to control, reuse, modify, and reengineer the cells’ structure and function, and it is expected to enable engineers to effectively use the biological cells as programmable substrates to realize Bio-NanoThings as biological embedded computing devices [2]. DNA sequencing and synthesis technologies, enabling the reading and writing of genetic code information in the DNA molecules of biological cells, are giving engineers an increasingly open access to the set of structural and functional instructions at the basis of life.
In particular, the engineering of synthetic biological circuits [5] through genetic code manipulation has enabled the programming of specifically designed functions to be executed by cells. A biological circuit is a set of genes that encode proteins and regulatory sequences, which link together the protein synthesis by mechanisms of mutual activation and repression. The functions today successfully developed via biological circuits range from AND and OR logic gates, to various types of tunable oscillators, toggle switches, and counters. The development of databases with characterized standard biological circuit parts with known functions and behaviors, e.g. BioBricks, and tools to combine them into more complex designs [6], are pushing synthetic biology to a future development similar to that experienced by integrated electrical circuit design in electronics. As a consequence, engineers will be soon able to gain full access to the functionalities of the aforementioned cells’ elements, and reuse cells and their features, without requiring an in-depth knowledge of biotechnology. One of the latest frontiers in synthetic biology is the development of artificial cells, enabled, among others, by tools from nanotechnology.
Artificial cells have minimal functionalities and structural components compared to natural cells, and are assembled bottom-up by encapsulating the necessary elements into either biological or fully synthetic enclosing membranes [7]. Artificial cells can therefore contain genetic information, the related molecular machineries for their transcription, translation, and replication, and all the required specialized molecules and structures. Artificial cells are expected to enable a more agile and controllable use of synthetic biological circuits by removing all the additional complexity of natural cells that are not necessary to perform the designed functions.
Although still in its infancy, this technology has been successfully applied, e.g. for drug delivery, gene therapy, and artificial blood cell production, and it is expected to deliver ideal substrates for synthetic biology with a more predictable behavior. Although very promising, the aforementioned technologies have to provide solutions to major research challenges in biotechnology and engineering before being considered as reliable tools for the realization of Bio-NanoThings. Focusing on the engineering design viewpoint, a major challenge is to develop reliable mathematical and physical models, and computer simulation environments, able to capture the peculiar characteristics of the biological processes underlying engineered cells, such as intrinsic non-linear phenomena and processes with noisy outcomes. Moreover, engineered cells, similar to natural cells, reproduce and mutate, i.e. tend to randomly change parts of their genetic programs, and selectively evolve, i.e. tend to maintain the best mutations for their survival while reproducing, adding possible problems but also new degrees of freedom to the biological device designer.
Another challenge that needs to be considered is related to bioethics and security, since autonomously evolving engineered organisms could pose a threat to the natural ecosystems, and even become new pathogens. The recent development of “kill” switches into biological circuits, able to stop cell reproduction or trigger cell destruction upon an external command, is only partially addressing these problems.
Bio-NanoThings Communications
At the basis of the IoBNT concept there is the need for Bio-NanoThings to communicate with each other, and interact on the basis of the exchanged information. Since Bio-NanoThings stem from the engineering of biological cells, as detailed above, the natural environment is the main inspiration for studying communication techniques for IoBNT.
MOLECULAR COMMUNICATION IN NATURE
In nature, the exchange of information between cells is based on the synthesis, transformation, emission, propagation, and reception of molecules through biochemical and physical processes. This information exchange, recently classified in telecommunications engineering as MC [1], enables cells’ interactions and coordination of uni-cellular and multi-cellular organisms, populations, and multi-species consortia, and participates in most of the major cellular functionalities such as cell growth and proliferation.
MC in cells is based on the aforementioned signaling pathways, which are chains of chemical reactions that process information signals modulated into chemical characteristics, such as molecule concentration, type, and energy state, and propagate them from a source, or transmitter, to a destination or receiver [4]. Cell signaling pathways can be classified on the basis of the distance between source and destination into intracrine (source and destination are within the same cell), juxtracrine (source and destination are cells in contact with each other), paracrine (source and destination are in the vicinity of each other, but not in contact), or endocrine (source and destination are distant from each other).
An example of intracrine communication is given by the intracellular transport of molecules or molecule structures operated by cytoskeletal molecular motors. Molecular motors are intracellular specialized proteins able to convert the aforementioned ATP molecules into mechanical energy. The cytoskeletal molecular motors are able to bind to a particular cargo, such as vesicles enclosing sets of molecules, or whole cell organelles, attach to the microfilament structures that compose the cell’s skeleton, and crawl along them transporting the cargo from the nucleus to the membrane of the cell and vice versa.
The exchange of molecules, such as calcium ions Ca2+, between two cells connected by communicating junctions in their membrane, is an example of juxtacrine communication. Several examples in nature, such as the signaling during a cardiac contraction happening between muscular cells, or myocytes, show how a small quantity of molecules can flow by diffusion between neighboring cells, and be responsible for synchronizing coordinated actions.
Bacteria show several means of communication in nature, such as the paracrine communication underlying the emission of signaling molecules called autoinducers by members of a population. In this process, called bacterial quorum sensing, the autoinducers diffuse within the intercellular space and, upon reception, allow the bacteria to estimate the population density, and have a correlated response, such as the production of specific types of proteins. Bacteria can also exchange specific DNA molecules, i.e. plasmids, via direct contact, through a process called conjugation, and carry the plasmids to other distant bacteria within the intercellular space by swimming according to chemical trails, with a process called chemotaxis.
In multicellular organisms, an example of endocrine communication is realized through signaling molecules called hormones that are emitted from cells composing glands, propagate through the circulatory system, and are received by the cells of distant organs, where they elicit specific responses, such as increased cell growth and reproduction.
CHALLENGES IN ENGINEERING MOLECULAR COMMUNICATION FOR IOBNT
Within the IoBNT, Bio-NanoThings are expected to interact with each other by exchanging various types of information, e.g. synchronization signals, values of sensed chemical/physical parameters, results of logical operations, and sets of instructions and commands. The engineering of the communication techniques to support these interactions in the biological environment has to stem from solutions found in nature, such as those described above.
One of the major challenges is to understand how these natural solutions can be controlled, modified, or reengineered for the transmission of information that can be different from the natural. By stemming from the aforementioned tools that are being developed in synthetic biology and nanotechnology, engineers have recently started to analyze several different possibilities to realize MC systems, either by genetically reprogramming cells’ behaviors within their natural communications [8], or by developing totally new artificial communication systems by assembling natural biological components [9].
Examples of MC systems that have been envisioned so far can be classified on the basis of the distance range that they are expected to cover from transmission to reception. For example, the control of juxtracrine communications through the genetic programming of biological cells can enable the engineering of networks where Bio- NanoThings are in contact with each other, e.g. when organized in a tissue or biofilm [10]. This MC technique, usually referred to the aforementioned Ca2+ exchange, shown in Fig. 2a, covers distances proportional to the thickness of cell membranes, and it can be considered as very short range (tens to hundreds of nm) MC. The aforementioned cytoskeletal molecular motors can be considered for the realization of MC in the short range (nm-mm) [11], as shown in Fig. 2b, to cover intracrine Bio-NanoThings communications.
Communication engineers have also combined models of the bacterial conjugation and chemotaxis processes described above to theoretically study a possible artificial MC system, which can be considered, according to the known chemotaxis characteristics, as covering the medium range (mm-mm) [9]. In particular, the information is represented into DNA molecules, i.e. plasmids, which are loaded at the transmitter location into bacteria and extracted from the same bacteria at the receiver through a conjugation process.
These bacteria are able to swim by chemotaxis toward the receiver, by following the receiver’s release of specific molecules, i.e. chemoattractants, as shown in Fig. 2c. An example of long range (mm-m) MC system has been envisioned by stemming from the hormonal communication within the human endocrine system [12], as shown in Fig. 2d. From the telecommunications engineering perspective, one of the main challenges stands in mapping MC into the classical elements of an engineered communication system, and in the use of tools from systems and information theory with the final goals of modeling and analyzing the main telecommunication characteristics and performance, such as range, delay (latency), capacity, throughput, and bit error rate [13].
The knowledge of these characteristics will then allow for the comparison and classification of possible different techniques to realize MC for different IoBNT application scenarios, and the optimization of their design and realization. Examples of the aforementioned mapping are shown in Fig. 3, where the main processes involved in each MC system described above are divided into communication elements as follows. Encoding and decoding are related to how the information to be transmitted is represented into one or more molecule characteristics, such as sets of particular molecule types and numbers (molecular motors and hormonal communication), composition of biological macromolecules, such as DNA plasmids (bacteria conjugationchemotaxis), or released molecule concentration (Ca2+ exchange).
Transmission and reception involve the chemical and physical processes to initiate the propagation of molecules, e.g. the encapsulation into vesicles for molecular motor transportation, the release of molecules in a fluid, such as the blood stream, or through a junction between two adjacent cells, or the release of bacteria upon the presence of chemoattractant molecules in the environment.
Finally, the propagation is concerned with the mobilization of the information-bearing molecules from the location of the transmitter to the receiver, such as through molecular motor crawling along microfilament structures, diffusion through membrane junctions, diffusion and advection in the blood stream, and bacterial chemotaxis toward the chemoattractant source (receiver).
While a great amount of the literature within the MC field has been devoted to the modeling and analysis of the aforementioned systems through simplifying assumptions, which increase the mathematical tractability of the underlying physical and chemical phenomena, there is still a long way to go for a communication engineer to fully understand how to design realistic MC systems for IoBNT communications.
The main challenges are given by the conversion of these simplified models to more realistic scenarios. For example, the free diffusion models considered so far in MC engineering for the propagation and reaction of molecules in the intracellular environment, e.g. in Ca2+ communication, have to be revised to include more realistic phenomena, such as the effect of high concentrations of macromolecules, e.g. proteins, called macromolecular crowding. Another example is given by the endocrine propagation, so far considered for a small subset of well-defined blood vessels, where models should take into account not only the whole average physiology of the human cardiovascular system, but also that the specific characteristics of each individual can result in very different propagation dynamics.
Also, the models of bacteria chemotaxis used so far in MC engineering are only based on the behavior and properties of single bacteria and in-vitro environments, where in fact more realistic environments, such as within the human body, and the fact that bacteria can replicate and proliferate dynamically and interact within multispecies consortia, should be taken into account. Other challenges for the development of reliable analytical tools for MC engineering are given by the non-linear nature of many biochemical phenomena, and the presence of very different noise sources, such as genetic mutations, compared to classical systems.
BIO-NANOTHING NETWORKS AND THE INTERNET


Within the IoBNT, Bio-NanoThings are expected to not only communicate with each other, but also interact into networks, which will ultimately interface with the Internet. To this end, the definition of network architectures and protocols on top of the aforementioned MC systems is an essential step for IoBNT development. A further challenge for the IoBNT is the interconnection of heterogeneous networks, i.e. composed of different types of Bio-NanoThings and based on different MC systems.
Finally, the realization of interfaces between the electrical domain of the Internet and the biochemical domain of the IoBNT networks will be the ultimate frontier to create a seamless interconnection between today’s cyber-world and the biological environment. In Fig. 4 we show a possible scenario where a complete IoBNT, composed of several networks based on different MC systems, is deployed inside the human body, and interfaces through a personal electrical device connected to the Internet to deliver intra-body status parameters (and receive commands and instructions) to (from) a healthcare provider.


CHALLENGES FOR REALIZING BIO-NANOTHING NETWORKS
While the engineering of computer networks is a well-established field, where several different solutions have been provided for many different technologies and application scenarios, the design of networks within the biological environment, and based on the MC paradigm as the physical medium, poses new challenges to the networking community. For example, molecular information generally does not follow predictable and definite propagation directions, as otherwise done by electromagnetic signals in classical communications [13].
The diffusion of molecules, the bacterial chemotaxis, and the filaments supporting molecular motors, tend to cover random patterns between source and destination. This and other peculiarities, such as the non-linear nature of many biochemical phenomena, make it particularly challenging to utilize classical techniques for regulating Bio-NanoThings access to shared media, such as fluids, addressing Bio-NanoThings, and designing information routing mechanisms, which are important basic aspects of computer networks.
As done for the MC systems, one possible solution will be to model, analyze, and reuse the mechanisms of interactions of multiple cells in nature, such as in bacteria populations [14] and multispecies consortia, or within the tissues of multi-cellular organisms, to relay the IoBNT information.
In this direction, a solution for the interconnection of heterogeneous Bio-NanoThing networks, based on different MC systems, might as well come from the natural way our body manages and fuses several types of information to maintain a stable, healthy status, or homeostasis [4]. These intra-body processes allow heterogeneous communications to occur at various scales, translating from intracrine communications within a cell, to juxtacrine communication within tissues, to endocrine communications between different organs. For example, the cells of the pituitary gland perform this type of translation by releasing hormones to body organs to control several processes, such as growth, blood pressure, temperature, and sleeping patterns, as a result of the reception of other hormones from the cells of the adjacent hypothalamic tissue.
Biological circuits based on these processes could effectively provide a set of genetic instructions that mimic the classical gateways between different subnets on the Internet. Figure 5a illustrates a general example of an artificial cell that translates the information encoded into molecules emitted from engineered bacteria into hormones that can be secreted into the circulatory system.
In this design, receptors would intercept the incoming molecules that, through a cascade of chemical reactions, would activate a biological circuit, which in turn would synthesize proteins able to trigger the necessary chemical reactions to produce the hormones.
CHALLENGES FOR BIO-CYBER INTERFACES
A bio-cyber interface is here defined as the set of processes necessary to translate information from the biochemical domain of Bio-NanoThing networks to the Internet cyber-domain, which is based on electrical circuits and electromagnetic communications, and vice versa. One of the main challenges for the realization of these interfaces stands in the engineering of chemical and physical processes able to accurately read the molecule characteristics where information is encoded, and translate them into the modulation of electromagnetic parameters. A possible solution in this direction might come from novel chemical and biological sensors enabled by nanotechnology, which promise unprecedented sensing capabilities [15].


These sensors are in general composed of materials characterized by electrical or electromagnetic properties that can be altered by the presence of determinate molecules or molecule complexes, such as biological receptors bound to molecules, and accordingly modulate the current in an electrical circuit. The major problems for using this sensing technology for IoBNT applications stand in their currently high latency, low selectivity, lack of standardized response, and, more importantly, unknown biocompatibility, which is considered next. Biocompatibility, intended here as the property of an engineered system of limiting its action on the biological environment exclusively to its intended function, without any unwanted alteration of biological parameters, is another challenge for the deployment of bio-cyber interfaces, especially for intra-body IoBNT applications as shown in Fig. 4. Given the limited size of the aforementioned nanosensors, and current promising research results in electromagnetic (EM) nanocommunications, we envision the possibility to develop bio-cyber interfaces by encapsulating biological nanosensors and EM nanocommunication units within the aforementioned artificial cells, as shown in Fig. 5b.
In this design, the biological nanosensor would be responsible for interfacing chemical and electrical domains, the EM nano-communication unit would wirelessly communicate with electrical devices outside of the biological environment, and the artificial cell would assure biocompatibility. However, a challenge lies in the ability to produce sufficient power for the wireless transmitter to emit electromagnetic waves that can propagate through the artificial cell membrane.
At the same time, approaches are also required to harvest energy for the transmitter unit from within the cell. Another alternative is to push the electrical/ EM domain at the physical interface between the biological environment and the external world, such as the skin for intra-body IoBNT applications. In this direction, electronic tattoos, similar to those based on radio frequency identification (RFID) technology, which allow users to authenticate devices within close range, could incorporate a bio-cyber interface able to sense bio- chemical information from cells on the epidermis, sweat glands, or nervous terminations, and communicate it wirelessly to nearby external electronic devices.
FURTHER CHALLENGES
We now briefly mention some further challenges to be faced for the IoBNT development. The IoBNT enabling technologies discussed in this article could pose serious security threats if handled with malicious intent. A new type of terrorism, which we term as bio-cyber terrorism, could effectively take advantage of the numerous possibilities offered by the IoBNT to control and interact with the biological environment.


For example, Bio-NanoThings could be used to access the human body, and either steal personal health-related information, or even create new diseases. Moreover, new types of viruses could be created to hack into already deployed IoBNTs.
Research within the IoBNT field should necessarily address these problems by combining the security assurance methods applied to today’s computer networks with security solutions developed through evolution by nature, such as the human immune system. The realization of localization and tracking techniques within the IoBNT, in a similar way as realized in wireless sensor networks (WSNs), could enable important applications related, for example, to the monitoring of disease locations in the body or identification of the location and distribution of toxic agents in an environment.
One solution could come from the engineering of chemotaxis in Bio-NanoThings, based on the aforementioned capability of bacteria to localize and track sources of particular types of molecules, which could be, for example, biomarkers released by cancerous or infected cells. In line with the vision of “all-Things connected,” an ultimate goal is to interconnect the paradigms of IoBNT and IoNT to the IoT.
A challenge of incorporating nanoscale devices is the large quantity of information that will emerge, taking the challenges of managing “Big- Data” to a new level. Besides the increase in the quantity of data, new services will need to be designed to semantically map between different types of data that IoBNT and IoNT will feed to the IoT. New service discovery solutions will also be required to search deep into the biological environments and interact with engineered biological entities to actuate or collect information.
Conclusion
While the Internet of Things (IoT) is enabling the pervasive connectivity of real-world physical elements among themselves and to the Internet, the Internet of NanoThings proposes to push the limits of this concept to nanotechnology-enabled nanoscale devices, which can be easily concealed, implanted, and scattered in the environment. In this article we introduced the further concept of Internet of Bio-NanoThings, where synthetic biology and nanotechnology are combined to develop Things based on the control, reuse, modification, and reengineering of biological cells.
This article outlined the challenges that will be faced to realize these Things and, more importantly, to enable their communication and networking, with paradigm-shifting techniques for the fields of communication and network engineering. We believe that the IoBNT research field, while still in its infancy, will result in a game-changer technology for the society of tomorrow.
REFERENCES
[1] I. F. Akyildiz and J. M. Jornet, “The Internet of Nano-Things,” IEEE Wireless Commun., vol. 17, no. 6, Dec.2010, pp. 58–63.
[2] L. J. Kahl and D. Endy, “A Survey of Enabling Technologies in Synthetic Biology,” J. Biological Engineering, vol. 7, no. 1, May 2013, p. 13.
[3] I. F. Akyildiz, F. Brunetti, and C. Blazquez, “Nanonetworks: A New Communication Paradigm,” Computer Networks, vol. 52, no. 12, Aug. 2008, pp. 2260–79.
[4] D. L. Nelson and M. M. Cox, Lehninger Principles of Biochemistry, W. H. Freeman, 2005, pp. 425–29.
[5] C. J. Myers, Engineering Genetic Circuits, Chapman & Hall/CRC, Mathematical and Computational Biology Series, 2009.
[6] D. Baker et al., Engineering Life: Building A Fab for Biology, Scientific American, vol. 294, no. 6, June 2006, pp. 44–51.
[7] F. Wu and C. Tan, “The Engineering of artificial Cellular Nanosystems Using Synthetic Biology Approaches,” WIREs Nanomedicine and Nanobiotech, vol. 6, no. 4, July/Aug. 2014.
[8] M. Pierobon,“A Systems-Theoretic Model of a Biological Circuit for Molecular Communication in Nanonetworks,” Nano Communication Networks (Elsevier), vol. 5, no. 1–2, Mar.–June 2014, pp. 25–34.
[9] M. Gregori and I. F. Akyildiz, “A New NanoNetwork Architecture using Flagellated Bacteria and Catalytic Nanomotors,” IEEE JSAC, vol. 28, no. 4, May 2010, pp. 612–19.
[10] M. Barros et al., “Transmission Protocols for Calcium-Signaling-based Molecular Communications in Deformable Cellular Tissue,” IEEE Trans. Nanotechnology, vol. 13, no. 4, May 2014, pp. 779–88.
[11] M. J. Moore, T. Suda, and K. Oiwa, “Molecular Communication: Modeling Noise Effects on Information Rate,” IEEE Trans. Nanobioscience, vol. 8, no. 2, June 2009, pp. 169–80.
[12] Y. Chahibi et al., “A Molecular Communication System Model for Particulate Drug Delivery Systems,” IEEE Trans. Biomedical Engineering, vol. 60, no. 12, 2013, pp. 3468–83.
[13] M. Pierobon and I. F. Akyildiz, “Fundamentals of Diffusion-Based Molecular Communication in Nanonetworks,” Now Publishers Inc, ISBN-10: 1601988168, ISBN-13: 978- 1601988164, Apr. 2014, 164 pages.
[14] I. F. Akyildiz et al., “MoNaCo: Fundamentals of Molecular Nano-Communication Networks,” IEEE Wireless Commun. Mag., vol. 19, no. 5, Oct. 2012, pp. 12–18.
[15] C. R. Yonzon et al., “Towards Advanced Chemical and Biological Nanosensors – An Overview,” Talanta, vol. 67, no. 3, Sept. 2005, pp. 438–48.
BIOGRAPHIES
I. F. AKYILDIZ is Ken Byers Chair Professor in Telecommunications with the School of Electrical and Computer Engineering, Georgia Institute of Technology, Atlanta, the Director of the Broadband Wireless Networking (BWN) Laboratory and the Chair of the Telecommunication Group at Georgia Tech. Since 2013 he has been a FiDiPro Professor (Finland Distinguished Professor Program (FiDiPro) supported by the Academy of Finland) in the Department of Electronics and Communications Engineering at Tampere University of Technology, Finland. He is an IEEE Fellow (1996) and an ACM Fellow (1997). He has received numerous awards from IEEE and ACM. His current research interests are in nanonetworks, TeraHertz band communication networks, 5G cellular systems, and wireless sensor networks.
M. PIEROBON received the Ph.D. degree in electrical and computer engineering from the Georgia Institute of Technology, Atlanta, GA, in 2013, and his M.S. degree in telecommunication engineering from the Politecnico di Milano, Milan, Italy, in 2005. Currently he is an assistant professor with the Department of Computer Science & Engineering at the University of Nebraska- Lincoln. He is an editor of the IEEE Transactions on Communications. He is a member of IEEE, ACM, and ACS. His current research interests are in molecular communication theory for nanonetworks, communication engineering applied to intelligent drug delivery systems, and telecommunication engineering applied to cell-to-cell communications.
S. BALASUBRAMANIAM received his bachelor (electrical and electronic engineering) and Ph.D. degrees from the University of Queensland in 1998 and 2005, respectively, and the master’s (computer and communication engineering) degree in 1999 from Queensland University of Technology. He is currently a senior research fellow at the Nano Communication Centre, Department of Electronic and Communication Engineering, Tampere University of Technology (TUT), Finland. He was the TPC co-chair for ACM NANOCOM 2014 and IEEE MoNaCom 2011. He is currently an editor for IEEE Internet of Things and Elsevier’s Nano Communication Networks. His current research interests include bio-inspired communication networks and molecular communication.
Y. KOUCHERYAVY (evgeni.kucheryavy@tut.fi) is a full professor and lab director in the Department of Electronics and Communications Engineering at the Tampere University of Technology (TUT), Finland. He received his Ph.D. degree (2004) from the TUT. He is the author of numerous publications in the field of advanced wired and wireless networking and communications. His current research interests include various aspects of heterogeneous wireless communication networks and systems, the Internet of Things and its standardization, and nano communications. He is an associate technical editor of IEEE Communications Magazine and an editor of IEEE Communications Surveys and Tutorials.
Read full story here…