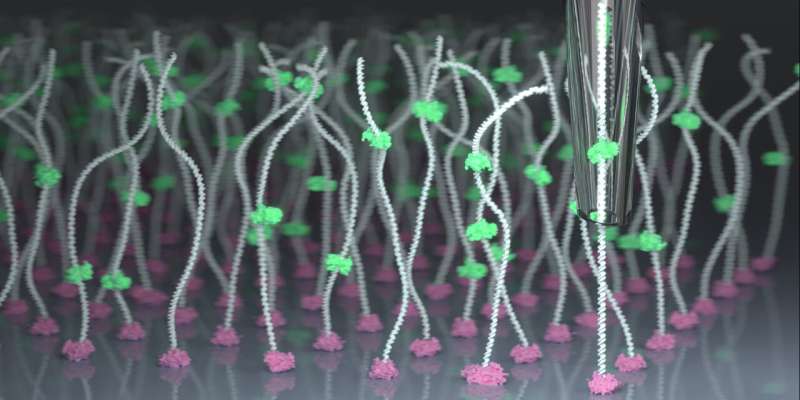
Aleksandra Radenovic, head of the Laboratory of Nanoscale Biology in the School of Engineering, has worked for years to improve nanopore technology, which involves passing a molecule like DNA through a tiny pore in a membrane to measure an ionic current. Scientists can determine DNA’s sequence of nucleotides—which encodes genetic information—by analyzing how each one perturbs this current as it passes through. The research has been published in Nature Nanotechnology.
Currently, the passage of molecules through a nanopore and the timing of their analysis are influenced by random physical forces, and the rapid movement of molecules makes achieving high analytical accuracy challenging. Radenovic has previously addressed these issues with optical tweezers and viscous liquids. Now, a collaboration with Georg Fantner and his team in the Laboratory for Bio- and Nano-Instrumentation at EPFL has yielded the advancement she’s been looking for—with results that could go far beyond DNA.
“We have combined the sensitivity of nanopores with the precision of scanning ion conductance microscopy (SICM), allowing us to lock onto specific molecules and locations and control how fast they move. This exquisite control could help fill a big gap in the field,” Radenovic says. The researchers achieved this control using a repurposed state-of-the-art scanning ion conductance microscope, recently developed at the Lab for Bio- and Nano-Instrumentation.
Improving sensing precision by two orders of magnitude
The serendipitous collaboration between the labs was catalyzed by Ph.D. student Samuel Leitão. His research focuses on SICM, in which variations in the ionic current flowing through a probe tip are used to produce high-resolution 3D image data. For his Ph.D., Leitão developed and applied SICM technology to the imaging of nanoscale cell structures, using a glass nanopore as the probe. In this new work, the team applied a SICM probe’s precision to moving molecules through a nanopore, rather than letting them diffuse through randomly.
Dubbed scanning ion conductance spectroscopy (SICS), the innovation slows molecule transit through the nanopore, allowing thousands of consecutive readings to be taken of the same molecule, and even of different locations on the molecule. The ability to control transit speed and average multiple readings of the same molecule has resulted in an increase in signal-to-noise ratio of two orders of magnitude compared to conventional methods.
“What’s particularly exciting is that this increased detection capability with SICS may be transferable to other solid-state and biological nanopore methods, which could significantly improve diagnostic and sequencing applications,” Leitão says.
Fantner summarizes the logic of the approach with an automotive analogy: “Imagine you are watching cars drive back and forth as you stand in front of a window. It’s a lot easier to read their license plate numbers if the cars slow down and drive by repeatedly,” he says. “We also get to decide if we want to measure 1,000 different molecules each one time or the same molecule 1,000 times, which represents a real paradigm shift in the field.”
This precision and versatility mean that the approach could be applied to molecules beyond DNA, such as protein building blocks called peptides, which could help advance proteomics as well as biomedical and clinical research.
“Finding a solution for sequencing peptides has been a significant challenge due to the complexity of their ‘license plates,’ which are made up of 20 characters (amino acids) as opposed to DNA’s four nucleotides,” says Radenovic. “For me, the most exciting hope is that this new control might open an easier path ahead to peptide sequencing.”
More information:
Spatially multiplexed single-molecule translocations through a nanopore at controlled speeds, Nature Nanotechnology (2023). DOI: 10.1038/s41565-023-01412-4
Provided by
Ecole Polytechnique Federale de Lausanne
Citation:
Extreme DNA resolution: Spatially multiplexed single-molecule translocations through a nanopore at controlled speeds (2023, June 19)
retrieved 19 June 2023
from https://phys.org/news/2023-06-extreme-dna-resolution-spatially-multiplexed.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.










