It cannot be overstated how much society relies on fossil fuels which is why it is important to look for other fuel sources that are sustainable, less environmentally damaging, and just as useful. As South Africa moves forward with its ‘Just Energy Transition’ (JET), other fuel sources have become a critical issue. The South African National Energy Development Institute (SANEDI) has laid out the path to a carbon-neutral South Africa and part of that plan is the ‘flagship Coal to X’ programme funded by the Department of Science, Technology, and Innovation (DSTI).
According to SANEDI, the ‘Coal-toX’ programme “aims to demonstrate technology that can capture CO2 from the flue gas of coal-fired power plants and convert together with (green) energy to commercially relevant products.” This is an ambitious multifaceted project, and one of these facets is found in the University of Cape Town’s Faculty of Engineering and the Built Environment. Prof Michael Claeys, the director of the DSI-NRF Centre of Excellence in Catalysis, a specialised centre within UCT that focuses on chemical catalysis, particularly for the conversion of gas and liquid fuels, says “The background to this is the rising CO2 levels. CO2 levels are also globally dispersed which means that it is a whole planet process.” One of the major focuses for South Africa is producing greener fuel sources with an emphasis on the green hydrogen economy. Green hydrogen is hydrogen derived from renewable energy sources rather than coal, and Prof Claeys says, “Green hydrogen is a large field, and in our case at the Centre for Catalysis, it is an enabling technology.”
Power to liquid

Prof Michael Claeys, the director of the DSI-NRF Centre of Excellence in Catalysis, a specialised centre within UCT Synthetic fuels burn much ‘cleaner’ than fossil fuels, making them more efficient
The Fischer-Tropsch process in
action at Sasol’s Secunda Facility
“Power to liquids is using renewable energy and converting that to liquids, in our case hydrocarbons such as diesel for sustainable aviation fuel. says Prof Claeys, “we are combing green hydrogen and CO2 and running it through catalytic processes to make synthetic liquid fuel.” The latest data shows that South Africa produces 397 million tonnes of CO2 a year, Prof Claeys adds, “South Africa produces a lot of CO2 through coal, but that gets difficult to use because it is in low concentrations and high in contaminants. There are other sources such as steel and cement production, which account for 8% of global CO2 production. On a smaller scale, there are biogas plants that emit CO2.” Smaller scale CO2 emitters are important to Prof Claeys and the Centre for Catalysis as they present higher concentrations that can be combined with hydrogen made on-site via electrolysis using renewable energy which is then converted to liquid fuel. South Africa’s solar potential is especially promising in green hydrogen production. This puts South Africa’s ‘power to liquid’ in a position where the country can produce its own fuel as well as export to countries where renewable energy is scarcer. “There has been a lot of interest from Europe in South Africa’s green hydrogen and renewable potential,” adds Prof Claeys.
Catalytic processes

The Fischer-Tropsch process in
action at Sasol’s Secunda Facility
South Africa is well known for the Fisher-Tropsch process. Sasol has been using this process to great success on a large scale. This process starts with converting hydrogen and CO2 into water and carbon monoxide (CO), here more hydrogen is added converting CO into various hydrocarbons through a polymerisation reaction. While Sasol uses coal and natural gas for their carbon sources, Prof Claeys’s centre aims to use other sources on a smaller scale.

“While there are large-scale projects and other initiatives like direct air capture, the idea is to leave nothing untapped. Using biogas centres, cement and steel producers will have a significant impact.”
These synthetic fuels, specifically diesel, can be used in the maritime, agriculture, and aviation sectors. “While cars can be replaced with electric vehicles, flying is heavily dependent on hydrocarbon fuels because of its energy density, so the Fischer-Tropsch process will play an active role in the sustainable fuel market going forward.” Fischer-Tropsch reaction hydrocarbons might also require ‘hydro-cracking” where the long chains of hydrocarbons are ‘chopped’ into shorter chains to maximise the fuel yield.
Synthetic fuels
A fuel’s combustion is measured via a cetane number (C10), everyday diesel has a C10 number of 45-55 indicating impurities and inefficiency. In contrast, synthetic fuel produced in the Fischer-Tropsch process is extremely high quality, with little to no impurities, and can have a C10 number of 100. A result of the synthetic fuel’s purity is that it burns much cleaner too. “Natural diesel has aromatic compounds, impurities, that burn inefficiently and give off more harmful chemicals into the environment. Whereas synthetic fuels burn ‘cleaner,’ we are also producing fuels that are completely sulfur-free. Removing sulfur from the process and producing fuels with no sulfur is important for the purity and efficiency of the fuel,” says Prof Claeys. Synthetic fuels will be used more as South Africa, and the world moves away from coal and fossil fuels. Prof Claeys says that the growing demand and South Africa’s well-suited position make for a sustainable future where South Africa is a major player.
The catalysis centre
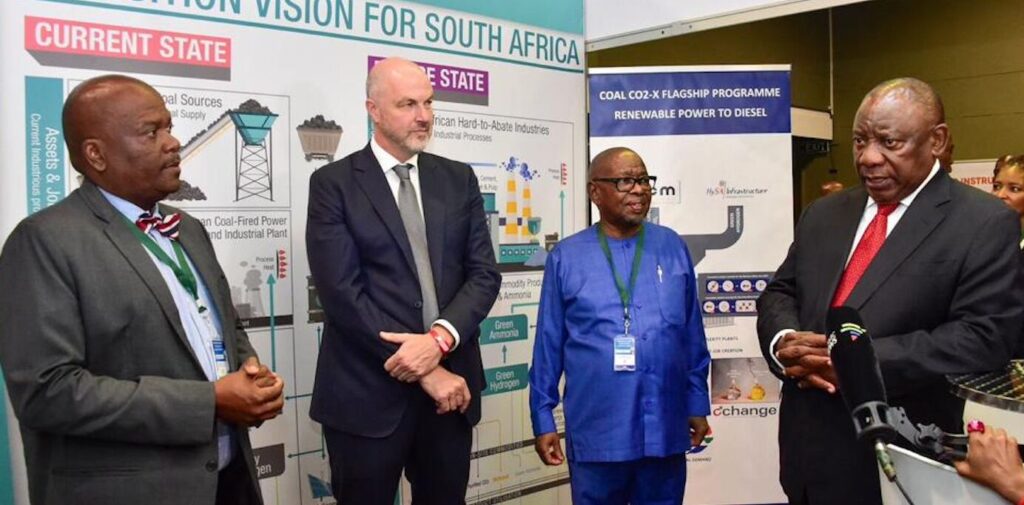
President of South Africa, Cyril Ramaphosa, getting the low down on the Fischer-Tropsch process
Prof Claeys has headed the DSI NRF, Center of Excellence for almost 20 years, showing a history of the process, and research in the country which it could leverage in the Just Energy Transition. The centre itself has graduated over 200 students, mostly Black and female and the centre aims not only to generate papers but patents. “The idea of generating patents was unusual for an academic centre at the time, but we took this quite seriously,” adds Prof Claeys. The centre has a lab-based demonstration unit, as well as experience in producing pilot plants. Prof Claeys says, “There is also an intention to have a larger unit that can be put onto a container for further demonstration. Go to various CO2 sites and run a demonstration and eventually commercialise the endeavour.”









