
Malaria remains one of the world’s deadliest diseases. Each year malaria infections result in hundreds of thousands of deaths, with the majority of fatalities occurring in children under five. The Centers for Disease Control and Prevention recently announced that five cases of mosquito-borne malaria were detected in the United States, the first reported spread in the country in two decades.
Fortunately, scientists are developing safe technologies to stop the transmission of malaria by genetically editing mosquitoes that spread the parasite that causes the disease. Researchers at the University of California San Diego led by Professor Omar Akbari’s laboratory have engineered a new way to genetically suppress populations of Anopheles gambiae, the mosquitoes that primarily spread malaria in Africa and contribute to economic poverty in affected regions.
The new system targets and kills females of the A. gambiae population since they bite and spread the disease.
Publishing July 5 in the journal Science Advances, first-author Andrea Smidler, a postdoctoral scholar in the UC San Diego School of Biological Sciences, along with former master’s students and co-first authors James Pai and Reema Apte, created a system called Ifegenia, an acronym for “inherited female elimination by genetically encoded nucleases to interrupt alleles.” The technique leverages the CRISPR technology to disrupt a gene known as femaleless (fle) that controls sexual development in A. gambiae mosquitoes.
Scientists at UC Berkeley and the California Institute of Technology contributed to the research effort.
Ifegenia works by genetically encoding the two main elements of CRISPR within African mosquitoes. These include a Cas9 nuclease, the molecular “scissors” that make the cuts and a guide RNA that directs the system to the target through a technique developed in these mosquitoes in Akbari’s lab. They genetically modified two mosquito families to separately express Cas9 and the fle-targeting guide RNA.
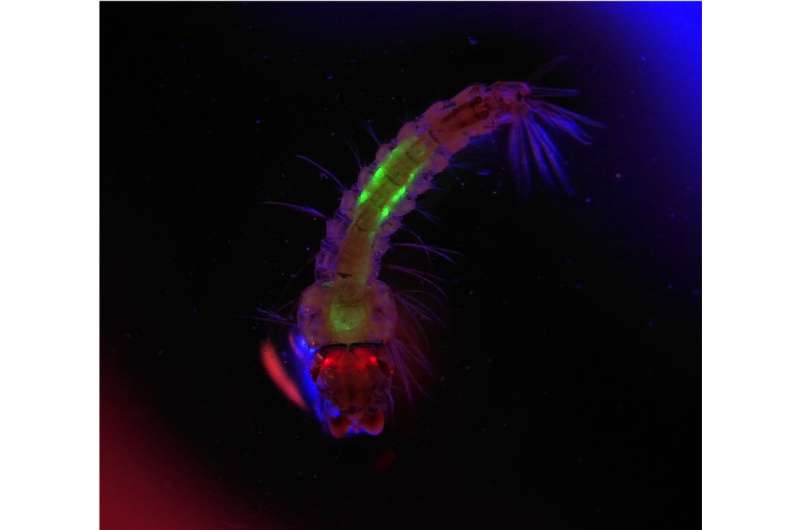
“We crossed them together and in the offspring it killed all the female mosquitoes,” said Smidler, “it was extraordinary.” Meanwhile, A. gambiae male mosquitoes inherit Ifegenia but the genetic edit doesn’t impact their reproduction. They remain reproductively fit to mate and spread Ifegenia.
Parasite spread eventually is halted since females are removed and the population reaches a reproductive dead end. The new system, the authors note, circumvents certain genetic resistance roadblocks and control issues faced by other systems such as gene drives since the Cas9 and guide RNA components are kept separate until the population is ready to be suppressed.
“We show that Ifegenia males remain reproductively viable, and can load both fle mutations and CRISPR machinery to induce fle mutations in subsequent generations, resulting in sustained population suppression,” the authors note in the paper. “Through modeling, we demonstrate that iterative releases of non-biting Ifegenia males can act as an effective, confinable, controllable and safe population suppression and elimination system.”
Traditional methods to combat malaria spread such as bed nets and insecticides increasingly have been proven ineffective in stopping the disease’s spread. Insecticides are still heavily used across the globe, primarily in an effort to stop malaria, which increases health and ecological risks to areas in Africa and Asia.
Smidler, who earned a Ph.D. (biological sciences of public health) from Harvard University before joining UC San Diego in 2019, is applying her expertise in genetic technology development to address the spread of the disease and the economic harm that comes with it. Once she and her colleagues developed Ifegenia, she was surprised by how effective the technology worked as a suppression system.
“This technology has the potential to be the safe, controllable and scalable solution the world urgently needs to eliminate malaria once and for all,” said Akbari, a professor in the Department of Cell and Developmental Biology. “Now we need to transition our efforts to seek social acceptance, regulatory use authorizations and funding opportunities to put this system to its ultimate test of suppressing wild malaria-transmitting mosquito populations. We are on the cusp of making a major impact in the world and won’t stop until that’s achieved.”
The researchers note that the technology behind Ifegenia could be adapted to other species that spread deadly diseases, such as mosquitoes known to transmit dengue (break-bone fever), chikungunya and yellow fever viruses.
More information:
Andrea L. Smidler et al, A confinable female-lethal population suppression system in the malaria vector, Anopheles gambiae, Science Advances (2023). DOI: 10.1126/sciadv.ade8903
Provided by
University of California – San Diego
Citation:
New genetic technology developed to halt malaria-spreading mosquitoes (2023, July 5)
retrieved 5 July 2023
from https://phys.org/news/2023-07-genetic-technology-halt-malaria-spreading-mosquitoes.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.









