When Ange Nzihou, an expert in converting society’s waste into valuable products, visited Princeton in 2022, he brought with him a technique to transform waste biomass into graphene, a material with many uses from batteries to solar cells. He knew his approach using a nontoxic iron catalyst offered advantages over existing methods relying on hazardous chemicals, precious metals or fossil fuels.
There was just one problem: Nzihou didn’t exactly know how the process worked.
“In my work as a chemical engineer, I’m often interested in the final properties of materials and how they can be applied to the real world,” said Nzihou, a distinguished professor of chemical engineering at IMT Mines Albi—CNRS in France who visited Princeton through the Fulbright Visiting Scholar Program. “But if you want to optimize the properties of the materials you produce, you have to understand what happens at the nano and atomic scales to bring about the transformation.”
That’s where Claire White, associate professor of civil and environmental engineering and the Andlinger Center for Energy and the Environment, came in to help.
As Nzihou’s faculty host, White contributed her expertise in the nano- and atomic-scale characterization of materials to uncover the mechanism that enabled iron to help convert waste biomass into graphene.
The result was not only two papers, the first published in ChemSusChem and the other in Applied Nano Materials, that detail the mechanism and promise of using iron as a catalyst to transform waste biomass, such as wood chips and other cellulose-rich biomass, into value-added carbon materials. It was also a launchpad for continued collaboration between the two groups, one that combined each group’s expertise to add new dimensions to their research programs.
A discovery of nano-scale proportions
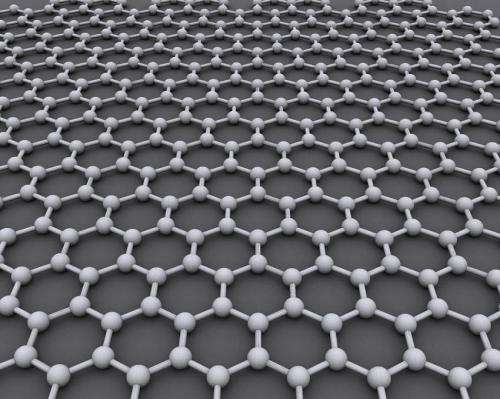
Graphene, a sheet of pure carbon just one atom thick, is commonly made via chemical vapor deposition, a process frequently used in the semiconductor industry to produce uniform coatings. However, Nzihou said chemical vapor deposition often hinges on hazardous chemicals and expensive technologies. Likewise, he said alternatives for graphene production typically employ toxic or cost-prohibitive materials, as well as the use of petroleum-based sources.
In search of an environmentally friendly way to produce graphene, Nzihou and White turned to underused sources of biomass as a starting material for the process. Unfortunately, most of that biomass is rich in cellulose, an abundant polymer found in the cell walls of plants. Cellulose has proven difficult to convert into highly ordered carbon materials such as graphene without the use of toxic or rare earth metal catalysts because of the structure and arrangement of its chemical bonds.
But Nzihou found that an iron oxide catalyst could do the trick. By inserting the iron into the biomass and heating it in an oxygen-limited environment through a process known as carbonization, Nzihou demonstrated it was possible to transform cellulose-rich biomass into a final material with extensive regions of ordered graphene sheets.
“Ange had shown that it was possible to use iron as a catalyst,” White said. “But the real question was in trying to understand how iron was providing this catalytic behavior.”
White turned to her expertise in atomic and nano-scale characterization for the answer. Using techniques such as X-ray total scattering, Raman spectroscopy, transmission electron microscopy, and magnetic measurements, the researchers found that over the course of the heating process, the iron oxide catalyst first broke down to form nanoparticles within the biomass. As the cellulose-rich biomass began to dissolve at higher temperatures, it precipitated as layers of graphene sheets onto the surface of the iron particles.
“We were actually able to observe this ordered shell of carbon atoms that formed around those iron nanoparticles during the process,” White said.
Interestingly, Nzihou and White found that a few larger iron nanoparticles supported more extensive regions of graphene formation than many smaller ones, a helpful clue that could inform future efforts to scale up the process of turning waste biomass into graphene. The researchers are also continuing to refine the process to increase the size of the pure graphene regions while reducing the number of defects in the final material.
“Now that we have an understanding of the mechanism, we can figure out how to improve the process and optimize the properties of the graphene sheets compared to the conventional chemical vapor deposition method, and even consider ways to scale it in the near future,” Nzihou said. “Because at the end of the day, our work is all about developing eco-friendly advanced carbon materials while closing the carbon loop and mitigating carbon dioxide emissions.”
A launchpad for fruitful collaborations
The researchers said that the project allowed them to leverage one another’s expertise to advance the field of sustainable carbon utilization, and the initial partnership has since dovetailed into multiple ongoing research projects.
“It’s been an exciting collaboration,” said White. “I would have never seen myself working on these sustainable carbon materials, but these projects with Ange have provided a prime opportunity to expand my work and add new dimensions to my research.”
For Nzihou, his time as a visiting Fulbright Scholar turned out to be only a preview of what is to come. He will return to the Andlinger Center in March 2024 as a Gerhard R. Andlinger Visiting Fellow to continue exploring ways to transform underused sources of biomass into advanced carbon materials with specific properties for applications ranging from agriculture to energy storage and CO2 sequestration.
With White, he plans to broaden the scope of his work by uniting the expertise of other Princeton faculty members such as Craig Arnold, Michele Sarazen and Rodney Priestley to develop a strategy for sustainable carbon utilization. He also aims to collaborate with the Princeton Plasma Physics Laboratory (PPPL) to explore the use of plasmas to power various production processes.
The first paper, “Synthesis and Growth of Green Graphene from Biochar Revealed by Magnetic Properties of Iron Catalyst,” was published Nov. 2022 in ChemSusChem. The second paper, “Iron Nanoparticles to Catalyze Graphitization of Cellulose for Energy Storage Applications,” was published Feb. 2023 in Applied Nano Materials.
More information:
Amel C. Ghogia et al, Synthesis and Growth of Green Graphene from Biochar Revealed by Magnetic Properties of Iron Catalyst, ChemSusChem (2022). DOI: 10.1002/cssc.202201864
Lina M. Romero Millán et al, Iron Nanoparticles to Catalyze Graphitization of Cellulose for Energy Storage Applications, ACS Applied Nano Materials (2023). DOI: 10.1021/acsanm.2c05312
Provided by
Princeton University
Citation:
Engineers reveal the secrets behind green graphene (2023, July 13)
retrieved 15 July 2023
from https://phys.org/news/2023-07-reveal-secrets-green-graphene.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.









