Excessive activation of inflammasomes is associated with various diseases, including gout, Alzheimer’s disease, atherosclerosis, and type 2 diabetes. Given the pivotal role of macrophages in both inflammasome activation and nanoparticle phagocytosis, the discovery of anti-inflammatory nanoparticles specifically targeting macrophages could more effectively modulated inflammatory response while minimizing off-target effects in other cell types.
However, the majority of currently reported nanomaterials have been found to promote rather than inhibit inflammasome activation.
A study published in the journal National Science Review demonstrated that nickel-cobalt alloy nanocrystals exhibit remarkable efficacy in suppressing the activation of three inflammasomes, namely NLRP3, NLRC4, and AIM2, in primary macrophages. Subsequently, the researchers employed two disease models, colitis and acute peritonitis, to evaluate the impact of nickel-cobalt alloy nanocrystals on treating inflammasome overactivation.
The findings revealed that nickel-cobalt alloy nanocrystals effectively ameliorated disease symptoms in mice in the colitis model, including mitigating weight loss, restoring colon length, and alleviating damage to the intestinal mucosal epithelium. Furthermore, in the acute peritonitis model, these nanocrystals significantly attenuated neutrophil chemotaxis within the peritoneal cavity of mice.
To confirm whether nickel-cobalt alloy nanocrystals require cellular internalization to exert their anti-inflammatory effects, the authors performed experiments using a widely used endocytosis inhibitor, cytochalasin D. Treatment with cytochalasin D significantly reduced the internalization of nickel-cobalt alloy nanocrystals by macrophages.
Moreover, inhibiting the internalization of the nanocrystals by macrophages led to a decrease in their anti-inflammatory effects, indicating that the anti-inflammatory action of nickel-cobalt alloy nanocrystals relies on their cellular uptake.
To investigate whether the anti-inflammatory effects of nickel-cobalt alloy nanocrystals are attributed to their geometric morphology or elemental composition, the authors synthesized nickel nanoparticles and cobalt nanoparticles under identical conditions as controls, which exhibited distinct morphologies compared to the nickel-cobalt alloy nanocrystals. However, both nickel nanoparticles and cobalt nanoparticles also significantly inhibited inflammasome activation.
Therefore, the authors attributed the inhibitory effect of nickel-cobalt alloy nanocrystals to the elemental composition rather than their geometric shape. These findings suggest that nanomaterials containing nickel and cobalt may offer opportunities for designing nano-drugs with anti-inflammatory properties.
Revealing the biological mechanisms underlying the action of nanomaterials is crucial for their potential medical applications. However, elucidating the biological mechanism by which these broad-spectrum anti-inflammatory nanocrystals inhibit inflammasome activation poses significant challenges using conventional biological experimental approaches.
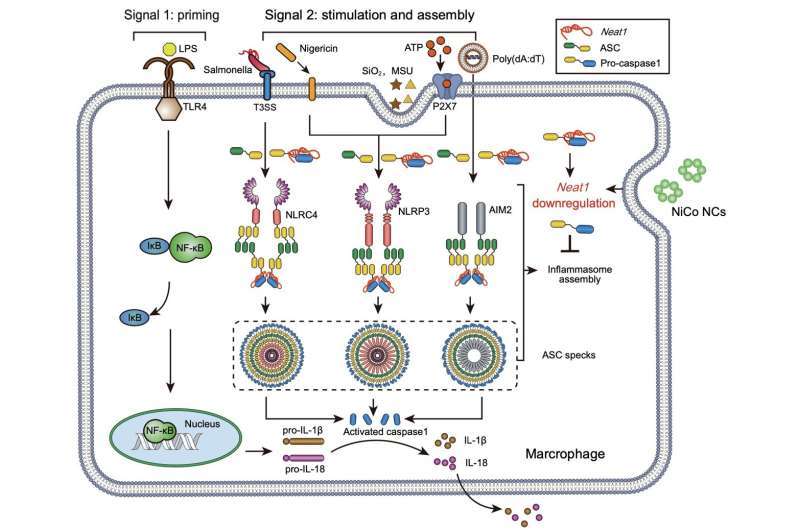
To address this, researchers conducted RNA sequencing and the assay for transposase-accessible chromatin with sequencing (ATAC-Seq), leading to the identification of a previously reported non-coding RNA, Neat1, known to be involved in inflammasome assembly. Following treatment with the nickel-cobalt alloy nanocrystals, the expression of Neat1 was significantly reduced.
Previous studies have demonstrated that downregulating Neat1 expression alone significantly inhibits the activation of NLRP3, NLRC4, and AIM2 inflammasomes. ATAC-Seq results revealed a significant reduction in the chromatin accessibility of the gene body and promoter regions of Neat1 in the nickel-cobalt alloy nanocrystal-treated group, suggesting that the inhibition of inflammasome activation by the nickel-cobalt alloy nanocrystals is achieved through the suppression of Neat1 transcription rather than promoting its degradation.
This study was collaboratively conducted by Dr. Shu-Hong Yu, Dr. Long-Ping Wen, and Dr. Kun Qu from the University of Science and Technology of China, together with Professor Yang Lu from Hefei University of Technology.
More information:
Jun Lin et al, Nickle-cobalt alloy nanocrystals inhibit activation of inflammasomes, National Science Review (2023). DOI: 10.1093/nsr/nwad179
Provided by
Science China Press
Citation:
Broad-spectrum inhibition of inflammasome activation by inorganic nanomaterials (2023, July 24)
retrieved 25 July 2023
from https://phys.org/news/2023-07-broad-spectrum-inhibition-inflammasome-inorganic-nanomaterials.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.










