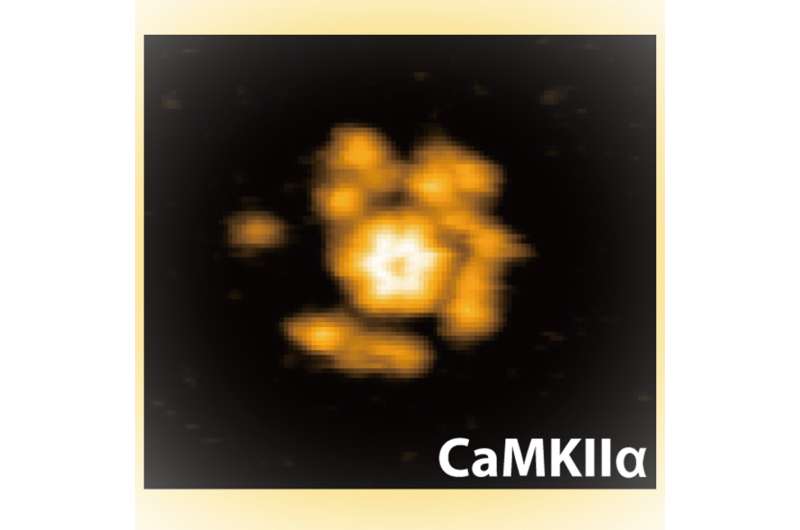
Researchers at Kanazawa University report in Science Advances high-speed atomic force microscopy experiments that show the structural and chemical changes in an enzyme thought to play a vital role in modulating the strength of neural connections.
Synapses connect neurons allowing the transmission of signals around the neural network. The strength of these connections varies—for instance strengthening or weakening depending on the signals received and how. This synaptic “plasticity” underlies learning and memory, and the Ca2+/calmodulin-dependent protein kinase II (CaMKII) is known to play a key role.
Previous studies have provided some clues to the mechanisms of CaMKII protein activity in these functions but no-one had seen these proteins in action. Now Hideji Murakoshi at the Graduate University of Advanced Studies and the National Institute for Physiological Sciences, and Mikihiro Shibata at Kanazawa University and their colleagues have used high speed atomic force microscopy (HS-AFM) to observe the structural dynamics of these proteins for the first time, not only in various states but in three different species.
CaMKII is common to a vast range of species from mammals like rats to older, non-mammalian species like the roundworms (C elegans) and hydra. In particular, certain structural features of the protein are particularly well preserved, including the kinase domain, the regulatory segment that inhibits the activity of the kinase domain and the hub domain. In addition, the protein has binding sites, phosphorylation sites and linker regions—however, the linker region shows a little more variability suggesting that its function and activation mechanisms are more bespoke for the different species.
Previous studies had suggested that the regulatory segment’s inhibition of the kinase domain is released when Ca2+/calmodulin binds to the regulatory segment. The activated kinase domains then phosphorylate each other, activity that persists even after the Ca2+/calmodulin becomes dissociated, which has been “hypothesized to be a form of molecular memory,” as the researchers describe in their report.
Murakoshi, Shibata and their colleagues studied the protein using atomic force microscopy, which feels topologies using a nanoscale tip like a needle reading a vinyl record, raster scanning the image plane to build up a picture of the sample structure. With HS-AFM, these images are collected quickly enough to record movies of how these structures change.
The researchers noted various measures of the proteins size and motion—the gyrus of rotation—as well as reactions such as kinase domain oligomerization (that is, where there is a limited level of polymerization to join molecules into chains) and phosphorylation—the addition of a phosphoryl group (PO3), which can activate enzymes like kinase.
They found that the kinase domain was quite mobile, although this decreased with Ca2+/calmodulin binding. The researchers explain this in terms of the binding stabilizing the protein into a particular helical structure called an α helix. They also note that the binding causes the protein to extend 3nm from the hub assembly, which they suggest may be what dissociates the regulatory segment from the active site in the kinase domain. The mobility then increases again with phosphorylation, which the researchers attribute to the protein losing some of the order of the helical structure.
Of the three species investigated—the rat, roundworm and hydra—Murakoshi, Shibata and their colleagues found that the kinase domain only oligomerized in the rat. In addition, in the rat, the protein was more resilient to phosphatase, which removes phosphate groups (PO4) from proteins.
“In conclusion,” they report, “Our findings provide a basis for a deeper understanding of the molecular mechanisms of CaMKIIα activation.”
More information:
Shotaro Tsujioka et al, Imaging single CaMKII holoenzymes at work by high-speed atomic force microscopy, Science Advances (2023). DOI: 10.1126/sciadv.adh1069
Provided by
Kanazawa University
Citation:
Experiments provide insights into the molecular mechanism for memory and learning (2023, July 7)
retrieved 7 July 2023
from https://phys.org/news/2023-07-insights-molecular-mechanism-memory.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.









