Induced pluripotent stem (iPS) cells have a great impact on biology and medicine, and they are expected to improve regenerative medicine. Since 2014, when a sheet of retinal pigment epithelial cells derived from iPS cells was transplanted into patients with age-related macular degeneration, clinical trials have been conducted with various cell types derived from iPS cells.
While iPS cells derived from healthy individuals have been used thus far, it is expected that transplantation therapy using iPS cells can be enhanced through genetic modification in the future.
Therefore, we addressed this possibility by utilizing a Fabry disease mouse model, as a proof of concept. Fabry disease is caused by the genetical deficiency of α-Galactosidase A (GLA), leading to the accumulation of its substrates such as globotriaosylceramide (Gb3) and globotriaosylsphingosine (Lyso-Gb3).
We previously developed an engineered enzyme, modified α-N-acetylgalactosaminidase (mNAGA), to cure Fabry disease by altering the substrate specificity of NAGA, which is a paralog of GLA, into that of GLA. Because mNAGA maintains the original antigenicity of NAGA, this modified enzyme has no immunological cross-reactivity with GLA, while having the GLA enzymatic activity.
In this study, which is published in Cell Transplantation, we tested whether transplantation of iPS cells secreting mNAGA by genome editing could supply the GLA activity in vivo.
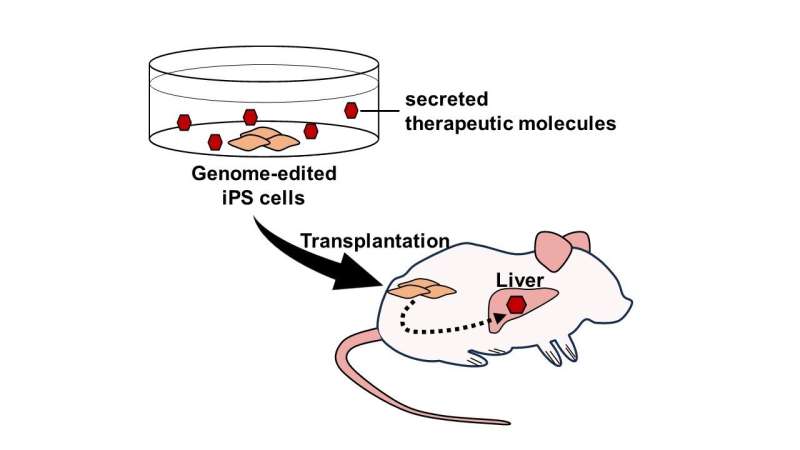
First, we generated iPS cells secreting mNAGA by TALEN-mediated knock-in to the AAVS1 site, a safe harbor locus. In addition, to exclude the possible immunogenic reactions caused by the endogenous GLA of iPS cells in patients, we disrupted the GLA gene by CRISPR-Cas9. When the Fabry model cardiomyocytes and fibroblasts with no GLA activity were co-cultured with mNAGA-secreting iPS cells, the GLA activity was restored by mNAGA-expressing cells in vitro.
Next, we transplanted the iPS cells secreting mNAGA into the testes of Fabry disease model mice. After seven or eight weeks, the GLA activity in the liver was significantly improved, although no recovery of the activity was observed in the heart, kidney, or blood plasma. We also quantified the amounts of Gb3 and Lyso-Gb3 in the liver, but there was no detectable reduction of the substrates.
Due to the limited amount of mNAGA secreted from the transplanted iPS cells, the GLA activity in the liver was not high enough to reduce Gb3 or Lyso-Gb3. However, in the future, it may be possible to enhance the amount of secreted mNAGA through genome editing. There is also the possibility to directly deliver mNAGA to organs and tissues that need the GLA activity. For example, transplantation of a cardiomyocyte sheet derived from iPS cells secreting mNAGA directly delivers mNAGA to the heart. Furthermore, while this study focused on Fabry disease, the same strategy can be applied to other diseases.
This study demonstrated the potential of cell therapy using genome-edited iPS cells secreting therapeutic molecules. These genome-edited iPS cells could serve as not only a resource for cell transplantation but also a drug delivery system.
More information:
Ittetsu Nakajima et al, In Vivo Delivery of Therapeutic Molecules by Transplantation of Genome-Edited Induced Pluripotent Stem Cells, Cell Transplantation (2023). DOI: 10.1177/09636897231173734
Provided by
Tokyo Metropolitan Institute of Medical Science
Citation:
Transplantation of genome-edited iPS cells delivers therapeutic molecules in vivo (2023, July 3)
retrieved 3 July 2023
from https://phys.org/news/2023-07-transplantation-genome-edited-ips-cells-therapeutic.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.